A guide to understanding boat batteries part 1, lead-acid

A wise sailor once told me this about boat electrics: As long as the lights came on at night, the engine starts in the morning, and the electric toilet flushes, all is well and other non-urgent matters can wait. This still holds true for me twenty-eight cruising years later, even given the hefty battery bank needed to achieve electrical nirvana on our 40-foot trawler Bliss. Unfortunately, however, we roasted that 1,855 amp hour bank earlier this year, and that meant a painfully expensive, unbudgeted replacement, plus no lights, no engine, no toilet — a real emergency. So having recently suffered through the selection process, I’d like to share what I know about the multitude of deep cycle high-performance battery choices.
In this two-part series, I’ll explore the workings, selection criteria, and maintenance of the two deep cycle marine battery technologies most used on pleasure craft. This part 1 is about various lead-acid batteries, and part 2 will focus on lithium-ion technology. My overall intention is not to recommend a specific battery type or manufacturer. As with most anything boat-related, there are compromises to be made when choosing batteries and no one correct answer. For example, Steve Dashew uses flooded batteries on his FPB 781 Cochese, Ben Ellison has Firefly carbon foam AGM batteries on Gizmo, Ben Stein hopes to try Mastervolt LFP lithium-ion batteries on Have Another Day, and we’ve gone with old fashioned gel cells on Bliss. The battery you choose will be a function of your vessel’s specific needs based on criteria like price, performance, capacity, size, weight, installation cost, charging method, maintenance, life expectancy, safety considerations, and installation location.
Unfortunately, batteries seem to have a mind of their own. For many of us who live with them they can seem moody and temperamental, they have good and bad days, and it seems impossible to understand how they work. Mostly we just get used to them and hope that they cooperate when we need them. To understand how to best care for your batteries, and to select the best ones for you, we need to dive into lead-acid and lithium-ion battery chemistries.
Let’s start with old fashioned lead-acid batteries, which have been around for over 100 years. In this category, we find flooded and sealed batteries. The flooded versions have caps that allow adding water while sealed maintenance-free batteries are well… sealed. Sealed batteries, also known as valve-regulated lead-acid (VRLA) batteries, fall into three groups, including gelled electrolyte (gel cells), absorbed glass mat (AGM), and carbon foam AGM. The same chemical principles apply to all of these battery types. Where they differ is their approach to addressing the physical deficiencies inherent in this technology.
Chemistry of lead-acid batteries
Understanding basic lead-acid battery chemistry is very valuable to understanding how they work and how to care for them. However, if you find this section daunting at first, reading it after the more familiar topics below may improve comprehension.
Lead-Acid batteries have the following basic components:
- A vessel where the electrochemical reactions take place.
- Two electrical poles where house wires are connected. One negative (anode) and one positive (cathode). Poles are often constructed of plates of alloys of lead, lead oxide, and antimony.
- An electrolyte, in this case sulfuric acid (also written as H2SO4).
- A solvent or medium. For flooded batteries, this is water (also written as H2O). As we will see shortly, water is also a reactive component (a reagent) in the electrochemical reaction. So in the case of flooded batteries, it does double duty. The medium must be able to allow ions (electrically charged species) to migrate between the poles.
When discussing batteries, the terms anode and cathode are often used to describe the poles’ polarity (plus or minus). Unfortunately, the corresponding pole depends on whether the battery is being charged or discharged. On discharge, the anode refers to the negative pole and the cathode to the positive one. On charging, the definition switches with the anode being the positive pole and the cathode being negative. Note that in this article, we use the terms relative to a discharging battery with anode referring to the negative pole unless otherwise stated.
Before proceeding, let me discuss some chemical nomenclature since we will need this to understand what makes batteries tick. Letters in chemical formulas stand for elements such as hydrogen (H), sulfur (S), oxygen (O), lead (Pb), lithium (Li), phosphorous (P), and carbon (C). These are combined into groupings to form molecules. A molecule may have one or more of the same element, which is indicated by a small subscript number following its letter designator. So a water molecule (H2O) contains two hydrogens and one oxygen atom. A sulfuric acid molecule (H2SO4) is comprised of 2 hydrogens, one sulfur, and four oxygen atoms. Digits in front of a molecule or atom, as in 2H2O, correspond to the number of molecules involved in the reaction. Finally, a superscript + or – (as in H+) corresponds to the charge of the entity. Charged entities are called ions, while neutral entities are called atoms or molecules. Hence, H2O is a water molecule, while SO4-2 is a sulfate ion with a negative charge of 2 (i.e., it has two extra electrons in its structure).
Electrons (e–) are negatively charged particles that bind atoms together to form molecules. Current is created when free electrons (electrons not bound to an atom or molecule) flow through a wire.
The following figures describe the electrochemical reactions that take place during battery discharge and battery charging. The anode (negative pole) is made of lead (Pb), the cathode (positive pole) is composed of lead oxide (PbO2), the electrolyte is sulfuric acid ( (H2SO4), and the medium or solvent is water (H2O).
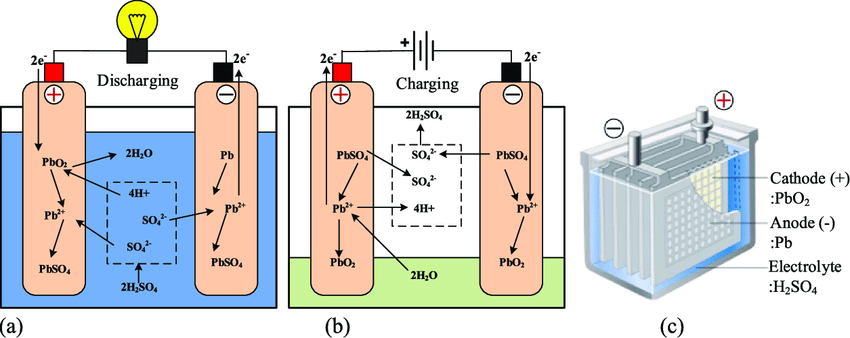
Let’s first look at the processes that take place when discharging the battery to power electronics on your vessel. At the anode (the negative pole of the battery) we have that lead (Pb) releases 2 electrons and a hydrogen ion (H+) and then binds to a sulfate ion (SO4-2) to form lead-sulfate (PbSO4). At the cathode, we have lead-oxide (PbO2) combining with 2 electrons, sulfuric acid, and the free hydrogen ion (H+) released at the anode to form lead sulfate (PbSO4) and 2 molecules of water (H2O). And since we have come this far (almost done) we can write this as
Anode reaction: Pb + HSO4– → PbSO4 + H+ + 2e–
Cathode reaction: PbO2 + 3H+ + HSO4– + 2e– → PbSO4 + 2H2O
The key takeaway here is that at the anode lead gives us two electrons that travel through your boat to do electrical work and end up at the cathode resulting in the creation of water and lead sulfate. This last statement is significant since it’s what generates the electricity you need and affects the longevity of your battery (more on this in a bit).
I won’t spend too much time on battery charging, which reverses the operation. The current flow is modified by your alternator, causing electrons to flow from the positive to the negative terminal regenerating lead (Pb) at the negative pole and lead oxide (PbO2) at the positive one.
An unwanted (and unfortunate) side reaction occurs during the charging cycle. Passing a current through an electrolyte causes water to disassociate into hydrogen (H2) and oxygen (O2). This process is known as electrolysis and releases hydrogen at the battery’s negative pole and oxygen at the positive pole.
Congratulations! This section ends the chemistry lesson. We can now discuss aspects of the reaction and how they affect the operation and maintenance of lead-acid batteries.
Lead-acid battery properties
Here are some takeaways that can be gleaned from the chemistry.
- Ions must be able to flow between the poles. Without ion flow, you can’t complete the electrochemical reaction, and the current will not be generated.
- Electrons flow from the negative to the positive pole, which is counter-current flow. We all know that in DC systems, minus corresponds to ground and that current flows from the + to – terminal. This is opposite the electron flow, which I have always found to be counter-intuitive! You can thank Benjamin Franklin for this convention, although he did create it about 100 years before the electron was discovered.
- Lead sulfate is formed at both the anode and cathode on discharge.
- The electrolysis of water is a (nasty) side reaction during charging.
- Discharging a battery requires electrons to flow from the anode to the cathode, which can happen internally, causing the battery to “self” discharge.
How does this all this affect your batteries?
Diffusion
Let’s look at ion flow first. It takes time for ions to flow through the medium from one pole to the other. This time factor manifests itself in total discharge rate and maximum charge rate for the battery. When discharging, fully charged batteries can produce a maximum amount of current known as cranking amps. The thicker (or more viscous) the medium the slower the ions flow through the substance and the lower the rate of electron or current production. So gel cells with more viscous medium have a slower rate of electron (current) generation than other lead-acid batteries. Grant you that poles do not need (and are not usually) rods stuck in water. Manufacturers make them in all shapes and sizes and play all kinds of tricks to get ions to flow quickly between the anode and cathode to yield better cranking amp performance for their batteries. However, all things being equal, the medium’s viscosity plays an essential factor in cranking amp performance.
Ion flow through the medium (diffusion) also affects the charge cycle. With a discharged battery, diffusion dictates the total current the battery can absorb during charge. This charging rate (max charge rate) is usually between 10-20% of the total capacity of the battery (or .1C to .2C where C represents the battery capacity). For a 100 ampere hour (Ah) battery, this means that the maximum initial current the battery can accept is about 10-20 amps. Pumping more current into the battery than it can accept causes electrolysis, which boils off the water releasing oxygen and hydrogen. Note that there is always some degree of electrolysis happening. However, its effect is minimal if the charging current is kept below the maximum acceptance threshold.
As battery charging proceeds the amount of lead sulfate at the poles decreases. Lead sulfate is converted back to lead at the anode and to lead oxide at the cathode. With decreasing concentrations of lead sulfate, the amount of current the battery can accept starts to drop. Thus, the battery voltage starts to rise if the charging current is not reduced as the battery charges. Note that temperature also increases, as does the rate of electrolysis. Think of a battery as a resistor where the excess current is dissipated as heat, hydrogen, and oxygen. As the lead sulfate is consumed, the resistance of the battery goes up, causing the voltage to increase as dictated by Ohms law (V = IR), which states that for constant current, there is a direct correlation between resistance and voltage. So for a constant charging current, the voltage increases as the resistance of the battery rises. Also, note that current running through a resistor generates heat, which causes the battery temperature to increase during charging.
Lead-acid batteries usually can accept their max charge rate through about 90% state of charge (i.e., 10% from full). Higher than 90%, the voltage starts to increase, so the charge current needs to be dropped. To charge a fully discharged battery using constant voltage and varying current usually takes about 60% of the time to charge 90% of the battery and 40% of the time to charge the remaining 10%. The old sailing adage “the closer you get, the slower you go” applies perfectly to battery charging! And because of this, we tend to stop the charging process well before the batteries are full when using our diesel engines to charge. And as we’ll see next, failure to fully charge lead-acid batteries is detrimental to their health.
Lead sulfate is your enemy
Lead sulfate is a battery discharge by-product that forms at both the anode and cathode. During battery recharge, the lead sulfate is consumed and turned into lead and lead oxide at the corresponding poles. When initially created, the lead sulfate is a gelatinous substance that reacts quickly, converting back to lead and lead oxide during the battery charge cycle. However, if left on the plates for long, lead sulfate crystallizes into a hard and much less reactive substance. In crystalline form, lead sulfate will not disassociate during charging, permanently binding up the otherwise active lead and reducing battery capacity. Not only does crystallized lead sulfate reduce capacity, but it also disrupts and slows the flow of ions between the poles reducing the charge/discharge efficiency of the battery. “Sulfated” batteries not only have less capacity but also have much lower cranking amp capacity. The bottom line, sulfate, is a double whammy when it comes to decreasing your battery’s performance. Unfortunately, the only way to prevent battery “Sulfation” is to discharge them lightly and recharge them to full capacity soon after use.
Equalization
Two kinds of lead sulfate crystals form during long discharge cycles. One type of crystal is inert, while the other can be forced to react by elevating the voltage during the charge cycle. This process is known as “equalizing” the battery and involves overcharging the battery at 15.5V while keeping an eye on the battery temperature, the battery’s specific gravity, and the electrolyte level. Heavy bubbling and gassing are expected. Equalization only works for flooded batteries and should be carried out reasonably frequently (Rolls recommends twice a year). If caught early enough, sulfation can be reversed for flooded batteries of good quality. However, leaving any lead-acid battery discharged for extended periods is a sure way to reduce its capacity or destroy it.
Some sealed batteries will allow for extended periods of higher voltage (usually considerably less than 15.5 volts) to rejuvenate battery capacity by breaking down lead sulfate. However, as a general rule, unless the manufacturer explicitly states that this can be done, the process should be avoided. Since electrolytes cannot be added to sealed batteries, manufacturer processes should be followed to the “T” when executing these recovery procedures.
Self-discharge
Electrons flow from the anode to the cathode when a battery discharges. Electrons (like sailors) are lazy (which is a good thing) and generally take the path of least resistance. This path is usually the wires that run through your boat, where the electrons are tricked into doing work. However, there is another path. In the absence of a direct external connection between the poles, electrons can flow through the electrolyte, causing the battery to “self” discharge. Self-discharge occurs for all battery chemistries and is typically about 5-10% of the battery capacity per month for flooded lead-acid batteries and (much) lower for sealed batteries.
Lead-acid battery take-away
The important take-away from all of this is that lead-acid batteries:
- Dislike being left in a discharged state
- Take a long time to reach full charge
- Will charge quickly at their maximum rated charge rating up to about 90% capacity and slowly thereafter.
- Self-discharge reasonably fast if left idle
- Will boil off electrolyte if charged at too high a current/voltage
- Will deteriorate with use over time. The more and deeper you discharge them the quicker they lose capacity
Early on, I was told without much explanation that a boat’s battery bank should be equal to no less than four times its total daily amp-hour (Ah) consumption. The battery bank should be recharged daily to no less than 90% capacity with a full charge once per week. And that philosophy (with good reason as we have seen) has served us well over the years.
Charging techiniques
There are several different charging strategies that can be used to charge lead-acid batteries.
A constant voltage is the simplest. Its what your car uses to maintain the starter battery. A voltage regulator is used to adjust the alternator’s current flow so that it never exceeds 13.35-13.5V. For trawlers crossing oceans, this works well. For those of us who spend long periods on the hook, not so much.
Sailboat and liveaboard motorists want to spend as little engine run time as possible charging batteries. Constant voltage regulation works fine for the first 60% of the battery, but it does very poorly for the last 40%. Long run times are required to charge your battery bank fully.
A better 3 phase technique is to use a smart regulator and a multi-stage process to allow maximum current to flow into the battery until the battery reaches a temporarily high voltage. This state is known as the bulk phase. The voltage, which is usually around 14.5V, is then maintained constant for a while. This state is known as the absorption or acceptance state. When the state of charge of the battery reaches about 95%, the regulator then reduces the voltage to a constant lower value. This 3rd state is known as the float.
The following plot depicts a 3 phase charge cycle.
More complex charging schemes (like the one below for sealed Victron gel batteries) are based on variations of the 3 phase charge cycle, but all pretty much work on the same principle. One important thing to note is that for all multi-step schemes to work, the voltage regulator must know the battery’s state of charge (SOC). Without this, the batteries will be either under or overcharged. See my article on Wakespeed’s WS500 regulator for a detailed discussion of determining a batteries SOC during charging.
Flooded vs. sealed batteries
Deep cycle lead-acid batteries come in two basic flavors, flooded and sealed (VRLA). The big difference between them is that flooded batteries use water as their medium with refill caps. In contrast, sealed batteries use a gel medium (or an otherwise solid medium such as glass matt). These batteries use a unique technology to recombine hydrogen and oxygen into water, neutralizing hydrolysis and making them maintenance-free.
There is a lot to like about flooded batteries as long as you are willing to maintain them. Maintenance means checking the water level periodically to ensure that the level never drops below the lead plates and occasionally put them through an equalize cycle. Deionized water should always be used when topping off batteries. Flooded batteries can be charged at higher voltages/current rates without damage as long as the water levels are maintained. Service life for them is quite good.
An often-overlooked advantage of flooded batteries is that since you have access to the electrolyte, a hydrometer can be used to measure the battery’s state of charge. The instrument measures the density of the electrolyte, which in turn yields the concentration of sulfuric acid. The higher levels of acid correspond to higher states of charge. A fully charged battery will test at 1.275-1.28, while a fully discharged battery will yield readings in the 1.140 range. Testing is as simple as removing a cap on the top of the cell and sucking electrolyte into the instrument.
There are a few drawbacks to flooded batteries:
- They require maintenance
- They must be installed upright. They will not operate on their sides or upside down.
- They can’t be submerged.
- They need to be installed in a well-ventilated area, especially if charged at elevated voltages and during equalization. They will generate hydrogen and oxygen, which can cause an explosion or fire.
The following chart shows the depth of discharge (DOD) vs. the number of cycles for Rolls flooded batteries obtained from the spec sheet for their 210 Ah 12 FS 185-HC battery.
So if you were to discharge this battery 35% and recharge every day, you could expect the battery to last about four years (1500 cycles). The chart should be used for guidance purposes only since the battery’s actual DOD life will vary on many factors, including the rate of discharge, length of time left in a discharged state, frequency of full charge, frequency of equalization, operating temperature, etc.
Note that the industry standard for battery failure is when it will not accept more than 20% of its original capacity. A 100 Ah battery, for example, fails when its capacity falls below 20% or 20 amp hours. Batteries don’t normally “just stop working”; the decline in capacity is usually a gradual process.
Unlike flooded batteries, there is no way to replenish the electrolyte in sealed lead-acid batteries. Sealed batteries use specialized technology that recombines hydrogen and oxygen generated by the electrolysis of water. But the technology has its limits. It can only handle moderate amounts of electrolysis. Thus, great care must be taken during the charge cycle to limit the amount of input current. Too much current boils off irreplaceable water reducing the capacity of the battery. Manufactures of sealed batteries recommend charging schemes specific to their batteries that minimize charging times while maximizing battery life expectancy. These schemes require intelligent multi-stage chargers, as discussed in my previous entry about the Wakespeed WS500.
The following graph depicts the charging profile recommended for the Victron gel batteries on Bliss.

The charge cycle starts by applying the maximum current recommended for the batteries by the manufacturer of 0.2C (e.g., 20 amps for a 100 Ah battery). Once the charge reaches 14.1-14.4 volts (the absorption voltage), the current is reduced to maintain a constant voltage. Once the batteries reach capacity (usually about 0.03C or 3% of its Ah capacity), then the current is reduced, and the voltage is allowed to drift down to a float voltage of 13.5-13.8V for up to 24 hours to complete the charge. If, after 24 hours, no current demands are placed on the battery, the charging current is reduced even further to drop the voltage to 13.2V for long term storage. The storage current offsets the battery’s self-discharge rate preventing the long term buildup of lead sulfate.
Note that the acceptance phase is particularly crucial for sealed batteries since the elevated charging voltage helps break down lead sulfate. Boaters with sealed batteries should make it a point to continue charging for a while once the acceptance voltage has been reached. Alternator current will start to drop in this phase, so the temptation is to shut down the engine terminating the charge cycle early. Early termination of the charge cycle is fine as long as the battery is brought to full charge soon.
There is a lot to like about sealed batteries once you can manage their finicky charging requirements. These include:
- Zero maintenance.
- No toxic or flammable gasses
- No acid spills or external pole corrosion caused by caustic gases and electrolyte overflow
- Some sealed batteries can be installed on their side, but check the manufacturer’s specifications.
- They can be submerged
- They usually have a lower discharge rate than flooded batteries. My gels self-discharge at 2% per month.
- Decent cranking amps
- Good longevity
AGM vs gel vs carbon foam batteries
Not all sealed batteries are created equal. There are very distinct differences in the manufacturing of their poles and electrolytic medium.
Gel batteries use a slurry of sulfuric acid and fumed silica; otherwise, they are quite similar to wet or flooded batteries except the internal plates use calcium instead of antimony to support the lead and lead dioxide.
Absorbent glass mat (AGM) batteries, on the other hand, use very thin glass fibers woven into a mat to hold the electrolyte. The plates in these batteries can be many different shapes but otherwise of similar composition to those found in flooded batteries or gels.
Carbon-Foam batteries use a carbon 3D honeycomb structure with thousands of micropores to fix the electrolyte and provide surface area for the lead and lead oxide required for electrochemical processes. This structure increases the surface area of lead, increasing efficiency while reducing lead sulfate’s harmful effects, proving exceptional longevity and high performance.
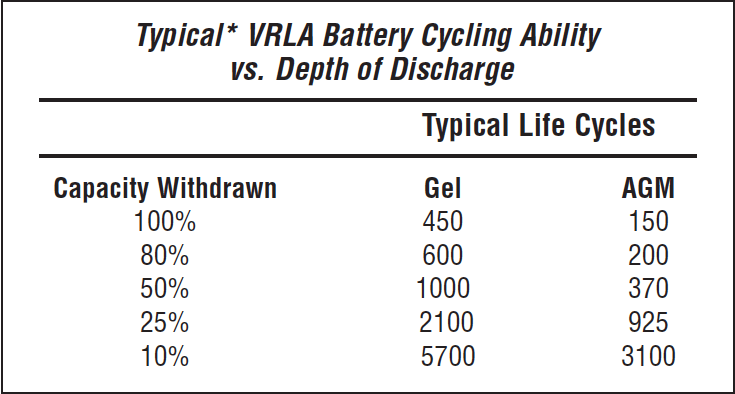
Let’s look at some charge cycle performance figures for AGM and gel batteries published by East Penn corporation for their batteries. East Penn is one of the world’s largest battery manufacturers selling batteries under many different brand names, including the West Marine AGM batteries. The following table shows the cycling ability for the East Penn VRLA batteries.
Note that the life expectancy drops with the depth of discharge, as with the flooded batteries. So “typically” you can discharge an AGM battery a total of 150 times before it fails, while gel batteries are usually more resistant at 450 total full discharges before failure.
Now note what happens if we limit the discharges to 25% of the bank capacity and charge the battery bank routinely, as previously discussed. You will see that for AGMs, you get 925 cycles or about 3.5 years of life if you discharge/recharge daily. On the other hand, gel batteries will last for 2100 cycles or 5.75 years before failing. So you almost double the life expectancy from gels than AGM when used to run the house. Which begs the question: Why are AGMs more popular than gels? I can only speculate here but suspect that most boats are small runabouts that use AGM batteries to start their engines in addition to running the house. Under these conditions, the AGMs perform well because they have higher cranking amps than gels and are never discharged deeply and recharged frequently. However, for a liveaboard who spends most of their time at anchor, gels would serve them batter.
Like flooded batteries, gels and AGM batteries last longer if cycled lightly and frequently changed.
Carbon foam batteries are another story. Let’s look at the technical spec for Firefly batteries as a comparison.
That is right… 900 full discharges before battery failure. And 5000 50% discharges before failure. This means 13.7 years of use when cycling and recharging the battery to 50%! Or 7.65 years if you consume 65% of the battery capacity daily. That is a crazy number of DOD cycles.
With carbon foam (as with lithium which we will examine in part 2) the 4x Ah/25% recommendation made previously for lead-acid batteries breaks down. Since carbon foam batteries have better longevity than flooded, AGM, or gel batteries your house bank can be much smaller, possibly even 2x or 1.5x your daily Ah consumption. This results in a physically smaller battery bank with a lower total cost per Ah.
Which begs the question: Knowing this, why did I choose gels over carbon foam batteries for Bliss? A good question indeed. Bliss was built in the early 1990s before the advent of newer battery technologies. Flooded, AGM, and gel batteries were state of the art at the time. Given that these batteries are made of lead, it was decided to use the batteries’ weight as ballast, in fact 2500 lbs of ballast. In other words, Bliss requires a huge battery bank both to keep her complex systems going, and to keep her upright.
So I could have purchased 2000 amp hours worth of carbon foam batteries, which would have given me approximately 45 years of service, or saved some money and bought gels with a life expectancy of 6 years. And there was also the issue of form factor. Firefly batteries come in two shapes, a 12V G31 100 Ah battery or a 4V L16 450 Ah battery. That would have been 20x G31 batteries or 12x L16 batteries. I couldn’t make these fit in my battery boxes, and rewiring would have been a nightmare! So gels it is. I currently have 7x 8D gels for the house (1855 Ah) and 1x 8D gel for the windlass and thruster that can be tied into the house. So really 2120 Ah. I also have a G31 gel for the engine starter battery, but we won’t count that.
Summing up
Batteries are complicated devices that require care in charging and maintenance. Every bank has its quirks and personality that need time and patience to get used to. I hope that with the knowledge gained here, you will be able to figure yours out and help it live a long and fruitful life.
In part 2 we’ll examine the chemistry and properties of lithium-ion batteries. Stay tuned…
















Nice article, waiting for part 2.
Lately I was sourcing information regarding best type of batteries in my situation. I am curious if these articles will solidify my thoughts.
I am wondering why you did not mention charging efficiency per type of battery? Maybe this is reserved for part 2 🙂
Good question.
There is discharge efficiency, and there is charging efficiency.
Charging efficiency corresponds to how many amps X must be generated by the alternator to store Y amps in the battery. Typically for lead-acid, the charge efficiency runs between 80-85%. So… to charge a 100 AmpHr battery, you will need to generate the equivalent of 115 (or so) Amp/Hr from the alternator keeping in mind that you want to observe the optimum charging curves discussed above. If you end up putting in more than the acceptance current, you increase electrolysis, decreasing your charge efficiency. So lots of actors at play. Again efficiency will vary as a function of the battery type, charge profile, and temperature.
On the other hand, discharge efficiency measures the amount of energy you get out of a battery based on its rated capacity. For lead-acid batteries, discharge efficiencies are very sensitive to the rate of discharge and temperature. If you discharge at the battery in 20 hours, you usually get about 100% of the rated capacity (not taking temperature variations into effect). Faster discharge rates will give you decreased capacity. So… say 80% at 4-hour rate or 60% at 1-hour rate.
The tech specs provided by the manufacturer provide all this information. Please take a look at
https://www.victronenergy.com/upload/documents/Datasheet-GEL-and-AGM-Batteries-EN.pdf
for my Victron gel batteries. You will find discharge efficiencies but no charge efficiencies. Just know those typical charge efficiencies for a lead-acid battery run about 80-85%… your mileage will vary.
Efficiencies for lithium-ion batteries is much higher for both charging and discharging. Typical discharging efficiencies are 100% for 20 hours, 99% for 4-hour rate, and 92% for 1-hour rates. Average charging efficiencies are 95% for these batteries.
More on this in part 2.
take care.
–luis
Luis, you state the “industry standard for battery failure is when it will not accept more than 20% of its original capacity.” Can you please reference where this standard is published?
I’ve reviewed many technical manuals and don’t remember seeing that 20% of original capacity clearly stated anywhere. I’ve seen anything from 50-80% of its original capacity in relation to warranty claims but can’t think of any that have gone as low as 20% before accepting a claim. When Eastpenn/Deka runs comparison tests with other manufacturers they run tests until original capacity drops to 50%. Trojan Battery states they consider the battery has failed when discharge runtime is less than 50% of the batteries’ rated capacity.
Thanks for the thorough article.
I don’t think you will be learning much about your batteries with a hygrometer. I suspect you mean a hydrometer.
I am a user of Odyssey AGM batteries in my MaineCat P-47. I use them for the house and the 3 starting batteries. The batteries are now 10 years old and still functioning fine. Odyssey claims 400 cycles of life at 80% discharge. This is twice what your article says for the “typical AGM”. When two brands of the same type battery have a 100% difference in in their life at 80% discharge it seems wrong to call your quoted number as “typical” of the type.
My house bank(630 ah @ 24 volts) has been severely discharged twice in its life. Once to less than 1 volt indicated and once to just over 9 volts indicated. Both due to user error when in winter storage. Anyhow the bank is still functioning fine and seems to still have about 90% of its original capacity and does a great job at supporting shorter term 4kva AC loads and long term 0,8 kva loads via the inverter. The house charging system is a Victron 5kva system from 2009/10 vintage with the correct charging profile specified by Odyssey when plugged into shore power or the genset is running and 200 amps of 24volt power from engine auxiliary alternators via a Balmar system again with the proper charging profile for the Odyssey batteries. In normal use the house bank is not discharged ever below 40% and usually not below 50%.
The three starting batteries still show as 95% charged, based upon the resting no load voltage check after 6 months of winter storage with no charging. One starting battery was discharged once to about 5 volts indicated and 2 of them discharged to about 6 volts indicated. The other advantage of AGMs (at least Odysseys but I think all) is they tend not to fail catastrophically but generally fail with a slowly reducing capacity over months so it is unlikely that the starting battery will work fine one day and not at all the next day as can happen with a flooded cell battery.
ANyhow, like so many things every case is different, but given my mistakes resulting in abuse to the batteries I am very happy with their long term performance/price/hassle balance.
Great article!
Thanks for this in depth analysis.
Looking forward to part 2.
-evan
Luis good article, however no mention of partial state of charge (psoc) vulnerability of agms with the exception of Firefly carbon Foam.
https://oceanplanetenergy.com/advanced-energy-storage-systems/firefly-energys-oasis-group-31/
Probably because this “exception” is not true, in perspective of the chemical side.
And the difference in this partial SOC aspect is mentioned in the article, I quote “This structure increases the surface area of lead, increasing efficiency while reducing lead sulfate’s harmful effects, proving exceptional longevity and high performance.”.
While it is reduced, it is still there (and could not be different, because the electrochemical reactions are the same).
Whil I cannot look ahead, probably in part 2 SOC and other aspects of batteries will be summarized, because lithium-ion batteries have their own issues in this aspect.
Luis –
Thank you for the excellent, quite thorough review given the length of the article (big topic!). Stressing the importance of limiting drawdown to 75%SOC is important to the liveaboard cruiser as well as periodic equalization to maintain battery health and longevity.
I thought that your table on cycle life for AGMs vs gels was slanted towards gels. I have 5 Lifeline 8D AGMs house batteries. Cycle life reported to be 2000 at a 25% depth of discharge.
Looking forward to part II.
Jelles
“Like flooded batteries, gels and AGM batteries last longer if cycled lightly and frequently changed.”
Was the last word meant to be “charged” instead of “changed”?
sorry about that… Yes. CHARGED is the what was intended here.
–luis
Note that lifeline AGMs are considerably different than EastPenn AGM batteries. They are milspec and have much better cycle life. Check out their user manual
https://321166-984045-raikfcquaxqncofqfm.stackpathdns.com/wp-content/uploads/2015/12/6-0101F-Lifeline-Technical-Manual-Final-5-06-19.pdf
they do close to 2000 cycles at 30% discharge and 1000 at 50% dischage. Not quite as good as the Carbon Foam batteries but very good none the less.
–luis